Tungsten information, including Technical Data, Safety Data and its high purity properties, research, applications and other useful facts are discussed below.hyScientific facts such as the atomic structure, ionization energy, abundance on Earth, conductivity and thermal properties are included.
Tungsten has the highest melting point of all the metallic elements and because of this has its first significant commercial application as the filament in incandescent light bulbs and fluorescent light bulbs. Tungsten is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity); metals in the form of foil, sputtering target, and rod, and compounds as submicron and nanopowder. Later it was used in the first television tubes. The first imaging equipment involved X-ray bombardment of a tungsten target. Tungsten expands at nearly the same rate as borosilicate glass and is used to make metal to glass seals. It is the primary metal in heating elements for electric furnaces and in any components where high pressure/temperature environments are expected, such as aerospace and engine systems. Tungsten is alloyed in steel to improve its ability to operate in high temperatures. Tungsten carbide is used in drill bits and cutting tools because it is one of the hardest commercial materials.. Tungsten forms compounds with calcium and magnesium that have phosphorescent properties and are used in the glass coatings for fluorescent light bulbs. Other tungsten chemical compounds are used in catalysts and lubricants. In reference to its density, Tungsten gets its name from the swedish words tung and sten meaning heavy stone.
Tungsten facts, including appearance, CAS #, and molecular formula and safety data, research and properties are available for many specific states, forms and shapes on the product pages listed to the left. Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Nanoparticles and nanopowders provide ultra high surface area which nanotechnology research and recent experiments demonstrate function to create new and unique properties and benefits.

Oxides are available in forms including powders and dense pellets for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Tungsten is available in soluble forms including chlorides, nitrates and acetates. These compounds are also manufactured as solutions at specified stoichiometries.
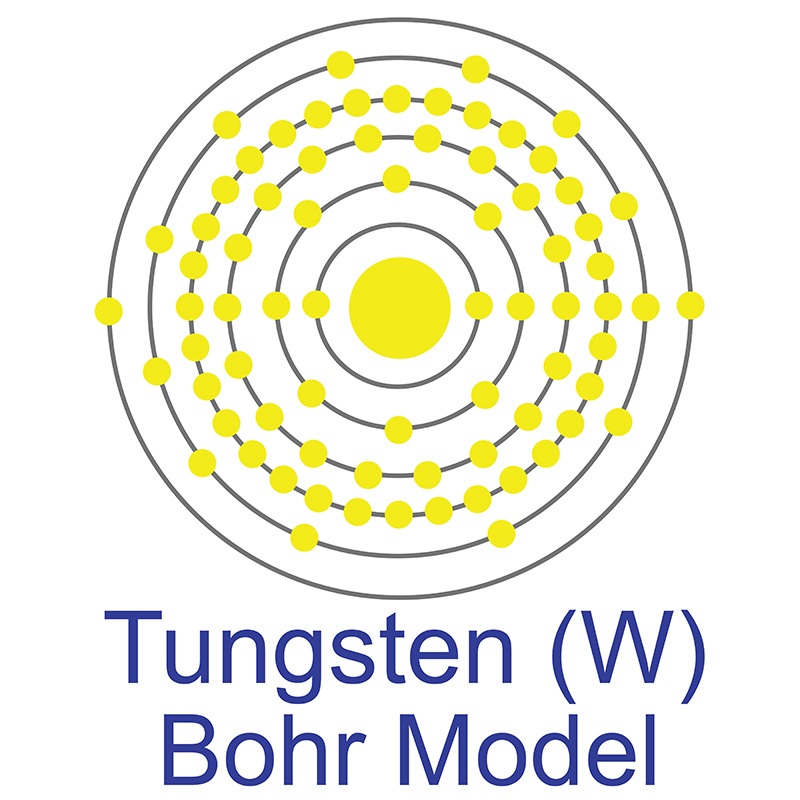
Tungsten is a Block D, Group 6, Period 6 element. The number of electrons in each of Tungsten's shells is 2, 8, 18, 32, 12, 2 and its electronic configuration is [Xe] 4f
14 5d
4 6s
2. In its elemental form tungsten's CAS number is 7440-33-7. The tungsten atom has a radius of 137.pm and it's Van der Waals radius is 200.pm. Tungsten is considered to be only mildly toxic.
All elemental metals, compounds and solutions may be synthesized in ultra high purity (e.g. 99.999%) for laboratory standards, advanced electronic, thin fillm deposition using sputtering targets

and evaporation materials, metallurgy and optical materials and other high technology applications. Information is provided for stable (non-radioactive) isotopes. Organo-Metallic Tungsten compounds are soluble in organic or non-aqueous solvents. See Analytical Services for information on available certified chemical and physical analysis techniques including MS-ICP, X-Ray Diffraction, PSD and Surface Area (BET) analysis.
Tungsten was first discovered by Fausto and Juan Jose de Elhuyar in 1783.
 tungstène tungstène |  Wolfram Wolfram |  tungsteno tungsteno |  Tungstênio Tungstênio |  wolframio wolframio |  Volfram Volfram |
Abundance. The following table shows the abundance of tungsten and each of its naturally occurring isotopes on Earth along with the atomic mass for each isotope.
| Isotope | Atomic Mass | % Abundance on Earth |
| W-180 | 179.946706 | 0.13 |
| W-182 | 181.948206 | 26.3 |
| W-183 | 182.950224 | 14.3 |
| W-184 | 183.950933 | 30.67 |
| W-186 | 185.954362 | 28.6 |
The following table shows the abundance of Tungsten present in the human body and in the universe scaled to parts per billion (ppb) by weight and by atom:
| | Typical Human Body | Universe |
| by Weight | no data | 0.5 ppb |
| by Atom | no data | 0.003 ppb |
Safety Data and Biological Role. The safety data for tungsten metal,
nanoparticles and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the left margin. Tungsten compounds have a small biological role in some enzymes.
Ionization Energy. The ionization energy for tungsten (the least required energy to release a single electron from the atom in it's ground state in the gas phase) is stated in the following table:
| 1st Ionization Energy | 758.77 kJ mol-1 |
| 2nd Ionization Energy | - kJ mol-1 |
| 3rd Ionization Energy | - kJ mol-1 |
Conductivity. As to tungsten's electrical and thermal conductivity, the electrical conductivity measured as to electrical resistivity @ 20 ºC is 5.4 μΩcm and its electronegativities (or its ability to draw electrons relative to other elements) is 1.7. The thermal conductivity of tungsten is 174 W m
-1 K
-1.
Thermal Properties. The melting point and boiling point for tungsten are stated below. The following chart sets forth the heat of fusion, heat of vaporization and heat of atomization.
| Heat of Fusion | 35.2 kJ mol-1 |
| Heat of Vaporization | 824.2 kJ mol-1 |
| Heat of Atomization | 848.1 kJ mol-1 |
Recent Research & Development for Tungsten
Gas nanosensor design packages based on tungsten oxide: mesocages, hollow spheres, and nanowires. Hoa ND, El-Safty SA. Nanotechnology. 2011 Dec 2;22(48):485503. Epub 2011 Nov 9. PMID: 22071572 [PubMed - in process]
Experimental hypothyroidism delays fEPSPs and disrupts hippocampal long-term potentiation in the dentate gyrus of hippocampal formation and Y-maze performance in adult rats. Seda Artis A, Bitiktas S, Taskin E, Dolu N, Liman N, Suer C. J Neuroendocrinol. 2011 Nov 9. doi: 10.1111/j.1365-2826.2011.02253.x. [Epub ahead of print] PMID: 22070634 [PubMed - as supplied by publisher]
Glycolaldehyde as a Probe Molecule for Biomass-derivatives: Reaction of C-OH and C=O Functional Groups on Monolayer Ni Surfaces. Yu W, Barteau MA, Chen JG. J Am Chem Soc. 2011 Nov 8. [Epub ahead of print] PMID: 22066750 [PubMed - as supplied by publisher]
[Bis-(4-methyl-1,3-thia-zol-2-yl-?N)methane]-tricarbonyl-dichlorido-tungsten(II). Strasser CE, Cronje S, Raubenheimer HG. Acta Crystallogr E Struct Rep Online. 2011 Oct 1;67(Pt 10):m1460. Epub 2011 Sep 30. PMID: 22065685 [PubMed]
Carbon nanotube composite coating of neural microelectrodes preferentially improves the multiunit signal-to-noise ratio. Baranauskas G, Maggiolini E, Castagnola E, Ansaldo A, Mazzoni A, Angotzi GN, Vato A, Ricci D, Panzeri S, Fadiga L. J Neural Eng. 2011 Nov 8;8(6):066013. [Epub ahead of print] PMID: 22064890 [PubMed - as supplied by publisher]
Use of carbon nanotubes and electrothermal atomic absorption spectrometry for the speciation of very low amounts of arsenic and antimony in waters. López-García I, Rivas RE, Hernández-Córdoba M. Talanta. 2011 Oct 30;86:52-7. Epub 2011 Aug 27. PMID: 22063510 [PubMed - in process]
Mechanism of W(CO)(6) sonolysis in diphenylmethane. Cau C, Nikitenko SI. Ultrason Sonochem. 2011 Oct 19. [Epub ahead of print] PMID: 22054911 [PubMed - as supplied by publisher]
Synthesis of macrocyclic natural products by catalyst-controlled stereoselective ring-closing metathesis. Yu M, Wang C, Kyle AF, Jakubec P, Dixon DJ, Schrock RR, Hoveyda AH. Nature. 2011 Nov 2;479(7371):88-93. doi: 10.1038/nature10563. PMID: 22051677 [PubMed - in process]
Combinatorial atmospheric pressure chemical vapor deposi-tion (cAPCVD); a route to functional property optimization. Kafizas A, Parkin IP. J Am Chem Soc. 2011 Nov 4. [Epub ahead of print] PMID: 22050427 [PubMed - as supplied by publisher]
Comparative evaluation of marginal adaptation between nanocomposites and microhybrid composites exposed to two light cure units. Sharma RD, Sharma J, Rani A. Indian J Dent Res. 2011 May;22(3):495. PMID: 22048600 [PubMed - in process]
Comparison of secondary neutron dose in proton therapy resulting from the use of a tungsten alloy MLC or a brass collimator system. Diffenderfer ES, Ainsley CG, Kirk ML, McDonough JE, Maughan RL. Med Phys. 2011 Nov;38(11):6248. PMID: 22047390 [PubMed - in process]
Vector potential photoelectron microscopy. Browning R. Rev Sci Instrum. 2011 Oct;82(10):103703. PMID: 22047299 [PubMed - in process]
Structural Effects Behind the Low Temperature Nonconventional Relaxor Behavior of the Sr(2)NaNb(5)O(15) Bronze. Torres-Pardo A, Jiménez R, González-Calbet JM, García-González E. Inorg Chem. 2011 Oct 28. [Epub ahead of print] PMID: 22035503 [PubMed - as supplied by publisher]
Accelerated electron beam induced breakdown of commercial WO(3) into nanorods in the presence of triethylamine. Dawson G, Zhou W, Blackley R. Phys Chem Chem Phys. 2011 Oct 27. [Epub ahead of print] PMID: 22030615 [PubMed - as supplied by publisher]
Multilayer chitosan-based open tubular capillary anion exchange column with integrated monolithic capillary suppressor. Huang X, Foss FW Jr, Dasgupta PK. Anal Chim Acta. 2011 Nov 30;707(1-2):210-7. Epub 2011 Sep 24. PMID: 22027141 [PubMed - in process]
Multispectral near-IR reflectance and transillumination imaging of teeth. Chung S, Fried D, Staninec M, Darling CL. Biomed Opt Express. 2011 Oct 1;2(10):2804-14. Epub 2011 Sep 15. PMID: 22025986 [PubMed]
Efficient Heterogeneous Epoxidation of Alkenes by a Supported Tungsten Oxide Catalyst. Kamata K, Yonehara K, Sumida Y, Hirata K, Nojima S, Mizuno N. Angew Chem Int Ed Engl. 2011 Oct 25. doi: 10.1002/anie.201106064. [Epub ahead of print] No abstract available. PMID: 22025368 [PubMed - as supplied by publisher]
Structural transformation of tungsten oxide nanourchins into IF-WS(2) nanoparticles: an aberration corrected STEM study. Leonard-Deepak F, Castro-Guerrero CF, Mejía-Rosales S, José-Yacamán M. Nanoscale. 2011 Oct 24. [Epub ahead of print] PMID: 22025289 [PubMed - as supplied by publisher]
Academic aspects of lunar water resources and their relevance to lunar protolife. Green J. Int J Mol Sci. 2011;12(9):6051-76. Epub 2011 Sep 19. PMID: 22016644 [PubMed - in process]
Evaluation of ocular hazards from 4 types of curing lights. Labrie D, Moe J, Price RB, Young ME, Felix CM. J Can Dent Assoc. 2011 Oct;77:b116. PMID: 22014874 [PubMed - in process]
|
Collected by Hanns CEO/
www.chinatungten.com
 Tungsten has the highest melting point of all the metallic elements and because of this has its first significant commercial application as the filament in incandescent light bulbs and fluorescent light bulbs. Tungsten is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity); metals in the form of foil, sputtering target, and rod, and compounds as submicron and nanopowder. Later it was used in the first television tubes. The first imaging equipment involved X-ray bombardment of a tungsten target. Tungsten expands at nearly the same rate as borosilicate glass and is used to make metal to glass seals. It is the primary metal in heating elements for electric furnaces and in any components where high pressure/temperature environments are expected, such as aerospace and engine systems. Tungsten is alloyed in steel to improve its ability to operate in high temperatures. Tungsten carbide is used in drill bits and cutting tools because it is one of the hardest commercial materials.. Tungsten forms compounds with calcium and magnesium that have phosphorescent properties and are used in the glass coatings for fluorescent light bulbs. Other tungsten chemical compounds are used in catalysts and lubricants. In reference to its density, Tungsten gets its name from the swedish words tung and sten meaning heavy stone.
Tungsten has the highest melting point of all the metallic elements and because of this has its first significant commercial application as the filament in incandescent light bulbs and fluorescent light bulbs. Tungsten is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity); metals in the form of foil, sputtering target, and rod, and compounds as submicron and nanopowder. Later it was used in the first television tubes. The first imaging equipment involved X-ray bombardment of a tungsten target. Tungsten expands at nearly the same rate as borosilicate glass and is used to make metal to glass seals. It is the primary metal in heating elements for electric furnaces and in any components where high pressure/temperature environments are expected, such as aerospace and engine systems. Tungsten is alloyed in steel to improve its ability to operate in high temperatures. Tungsten carbide is used in drill bits and cutting tools because it is one of the hardest commercial materials.. Tungsten forms compounds with calcium and magnesium that have phosphorescent properties and are used in the glass coatings for fluorescent light bulbs. Other tungsten chemical compounds are used in catalysts and lubricants. In reference to its density, Tungsten gets its name from the swedish words tung and sten meaning heavy stone.  Oxides are available in forms including powders and dense pellets for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Tungsten is available in soluble forms including chlorides, nitrates and acetates. These compounds are also manufactured as solutions at specified stoichiometries.
Oxides are available in forms including powders and dense pellets for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Tungsten is available in soluble forms including chlorides, nitrates and acetates. These compounds are also manufactured as solutions at specified stoichiometries.  and evaporation materials, metallurgy and optical materials and other high technology applications. Information is provided for stable (non-radioactive) isotopes. Organo-Metallic Tungsten compounds are soluble in organic or non-aqueous solvents. See Analytical Services for information on available certified chemical and physical analysis techniques including MS-ICP, X-Ray Diffraction, PSD and Surface Area (BET) analysis.
and evaporation materials, metallurgy and optical materials and other high technology applications. Information is provided for stable (non-radioactive) isotopes. Organo-Metallic Tungsten compounds are soluble in organic or non-aqueous solvents. See Analytical Services for information on available certified chemical and physical analysis techniques including MS-ICP, X-Ray Diffraction, PSD and Surface Area (BET) analysis. 

No comments:
Post a Comment